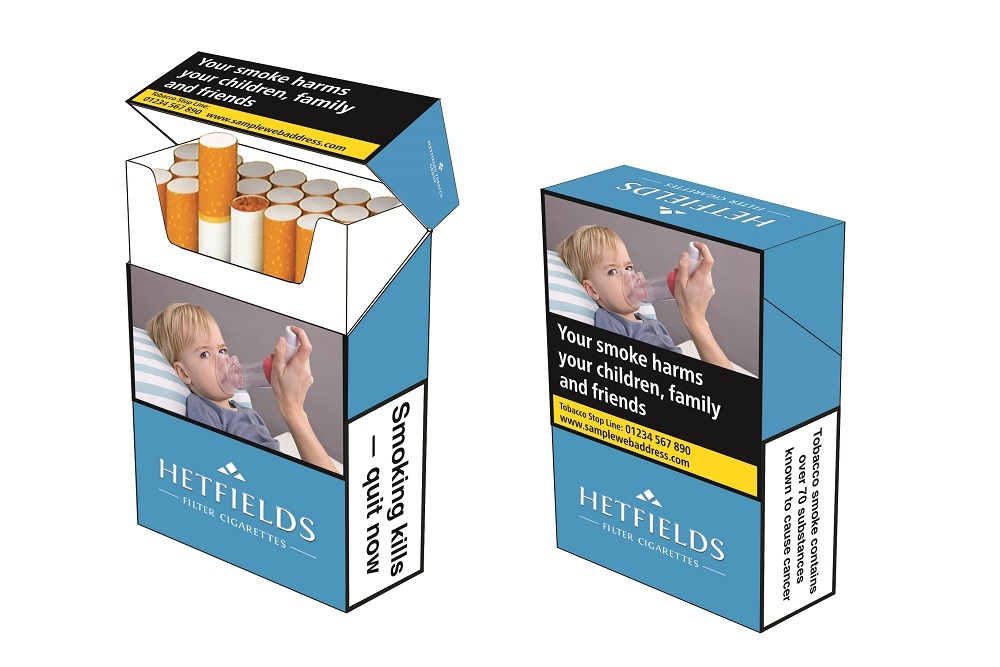
Health warnings
European Commission recently issued the following announcement.
In certain areas, the European Parliament and the Council have empowered the European Commission to adopt further legislative acts to aid the implementation process of Directive 2014/40/EU. This legislation takes the form of delegated or implementing acts which outline in more detail the rules and measures regarding tobacco and related products.
Implementation and delegated acts
The European Commission has adopted implementing legislation that lays down more detailed technical rules. This includes:
|
Implementation plan
The development of this secondary legislation involves, as appropriate, studies and involvement of Member States and other stakeholders.
This implementation plan serves to allow transparency towards stakeholders on how the Commission intends to prepare the implementing measures, as required by this Directive. It is of indicative nature.
Original source can be found here.

